
Schedule I drugs, substances, or chemicals are defined as drugs with no currently accepted medical use and a high potential for abuse. §802(32)(A) for the definition of a controlled substance analogue and 21 U.S.C. A controlled substance analogue is a substance which is intended for human consumption and is structurally or pharmacologically substantially similar to or is represented as being similar to a Schedule I or Schedule II substance and is not an approved medication in the United States. Please note that a substance need not be listed as a controlled substance to be treated as a Schedule I substance for criminal prosecution.

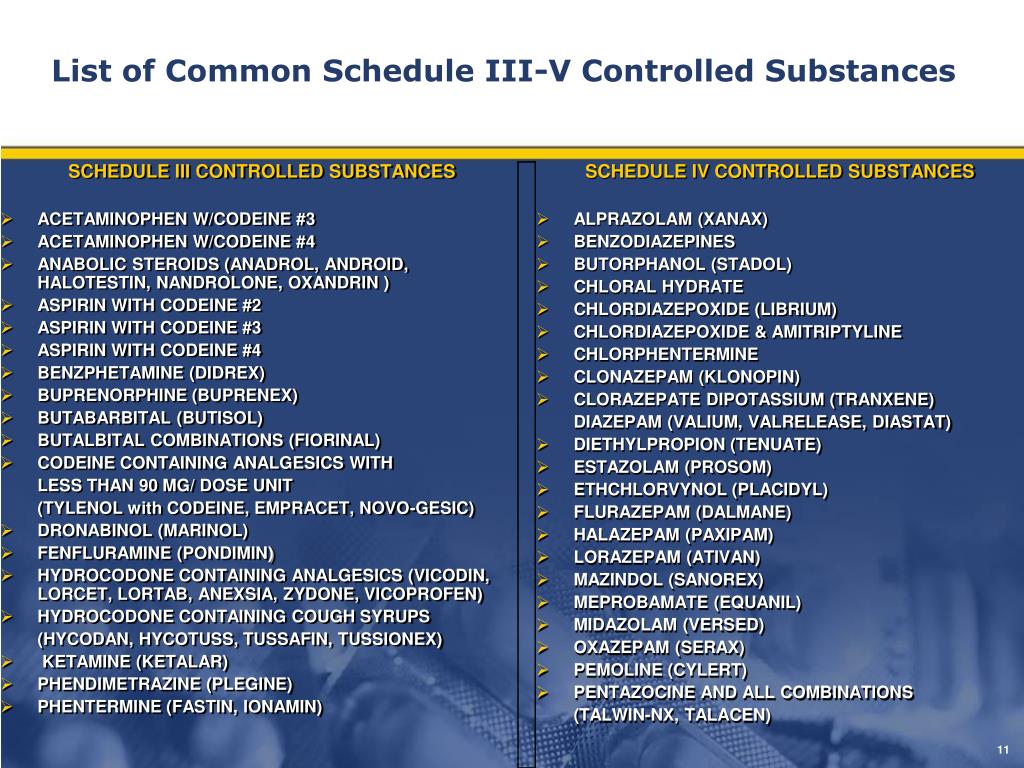
These lists are intended as general references and are not comprehensive listings of all controlled substances. These lists describes the basic or parent chemical and do not necessarily describe the salts, isomers and salts of isomers, esters, ethers and derivatives which may also be classified as controlled substances. A Listing of drugs and their schedule are located at Controlled Substance Act (CSA) Scheduling or CSA Scheduling by Alphabetical Order. As the drug schedule changes- Schedule II, Schedule III, etc., so does the abuse potential- Schedule V drugs represents the least potential for abuse. The abuse rate is a determinate factor in the scheduling of the drug for example, Schedule I drugs have a high potential for abuse and the potential to create severe psychological and/or physical dependence. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential.


 0 kommentar(er)
0 kommentar(er)
